The strategy of Pharmaceutical Industry and Government
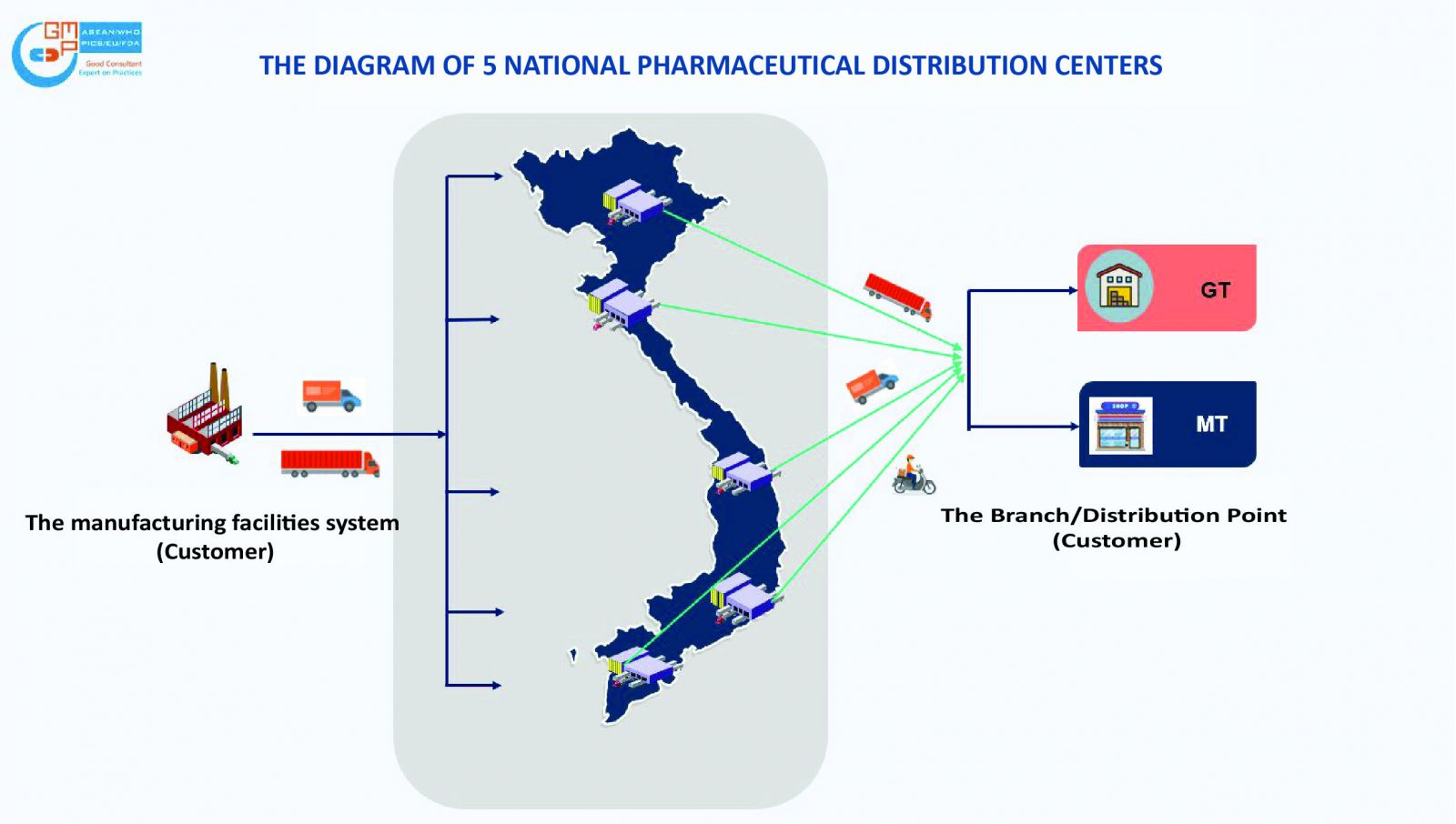
"The national development strategy for phamarceutical industry to 2020, vision to 2030" clearly indicates: 05 pharmaceutical distribution centers will be built in the Northern mountainous areas, the North Central, the South Central Coast-Central Highlands, the Southeast and Southwest region;
Decision No.376/QD-TTg dated March 17, 2021 on "Approving development program for the pharmaceuticals industry and domestically produced herbal until 2030 and vision to 2045" added: Investment on building and rearrangement of the distribution system, wholesale, retail, and domestical pharmaceutical transportation to be modern, professional and efficient. Encouraging application of EU-GSDP technical standards, building pharmaceutical storage warehouses and transport vehicles system with high technical standards.
The differences between Pharmaceutical Distribution Center and General Pharmaceutical Warehouse?
The Pharmaceutical Distribution Center is a modern, advanced model, that integrating the general warehouse following GSP regulations with a full range of Logistics services: Order processing, Shipping management, Supervision management, Testing/Packaging service,...
The value of Pharmaceutical Distribution Center
1. Customers (pharmaceutical distributors)
Financial benefits
- No need to invest in GSP warehouses, GDP facilities, means of transport, etc.
- No need to pay operating and maintenance costs
- Only pay rental cost that depends on the actual amount of goods for storage (m3/day or pallets/day)
- Minimize the number/cost of specialized and operating personnel
Convenience
- Do not waste time for building facilities, personnel systems, and processes, ..
- Professional and responsible partner
- Full range of Logistics service: Order processing, delivery, reporting, ...
Quality of service is guaranteed
- Guaranteed quality (Storage conditions - Online monitoring, Periodic reports)
- Professional packaging
- Meet the schedule, on-time delivery
Compliance with the Adminstrative Department requirements
- No need/Minimize reporting
- Conditions in compliance with requirements
2. Government Administration
- Convenient for centralized Quality Management
- Easily collect statistical data for forecasting and building strategy
3. Society
- Improve the drug quality
- Contribute to lower drug prices by reducing intermediate distributors.
4. Project Investor
- The pioneering, modern and sustainable service model
- Satisfied all of customers' expectations, even more than that.
- Bring high economic efficiency
5. Enterprise/Support services
- Development of transportation services that require temperature control and special storage
- Development of the production and service realted to Packaging, packaging materials, labeling, ..
GMPc Vietnam





















