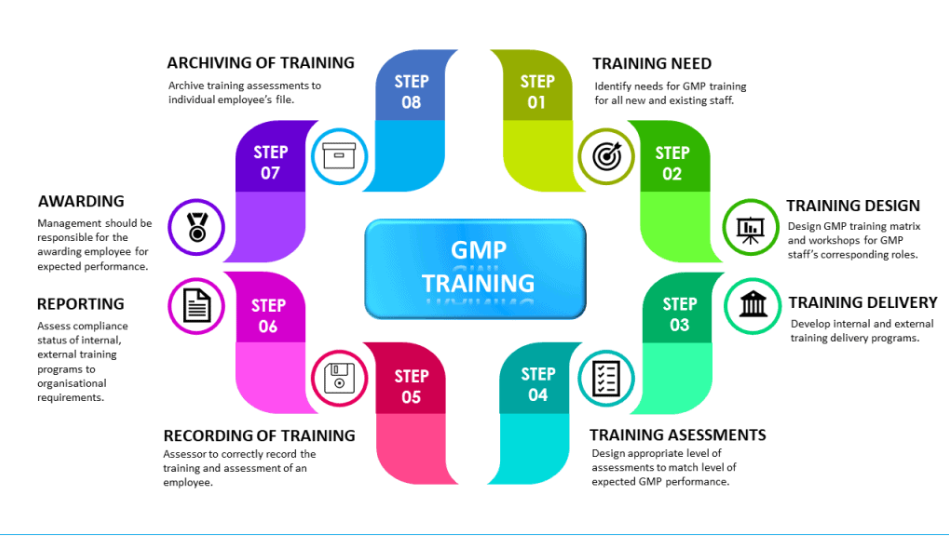
The development, implementation and execution of GMP training for employees can be varied depending on business operation and respective regulatory expectations. In principle, the GMP training program can be built around the following steps with clear objective and deliverables. The training program should start with training requirements, planning and design, delivery, assessment, record keeping and archiving stages.
Step 1: Training need
The senior managers along with subject matter experts should carry out the following analysis to properly identify the employee training needs.
Request feedback the department managers
Review feedback employee development need
Evaluate internal audit findings
Review FDA or customer audits findings
Review policies and plans that need to be complied against
Review compliance with in-house and regulatory requirements
Reviewing GMP breaches and incidents to assess trends
Identify organizational opportunities for training.
Step 2: Training design
At the step of designing the training program the management and subject matter experts should ask the following questions:
Who is to be trained?
What are the training topics or subject matter?
From where will training materials come?
Who would be the trainers?
Where to conduct training?
What is the cost of training?
What is the duration of training?
What are the schedules and priorities? Etc.
During the design phase the team should draw a skills training matrix for each role, listing the skills, reference training course against each employee who must be trained.
In the skills training matrix, the timeframe should be set by which employee must complete their training. The skills matrix may refer to written procedures, training modules or other training resources.
GMP Points can be designed to be earned by the employees through attendance at internal and external GMP Training courses, workshops, certifications etc.
Step 3: Training delivery
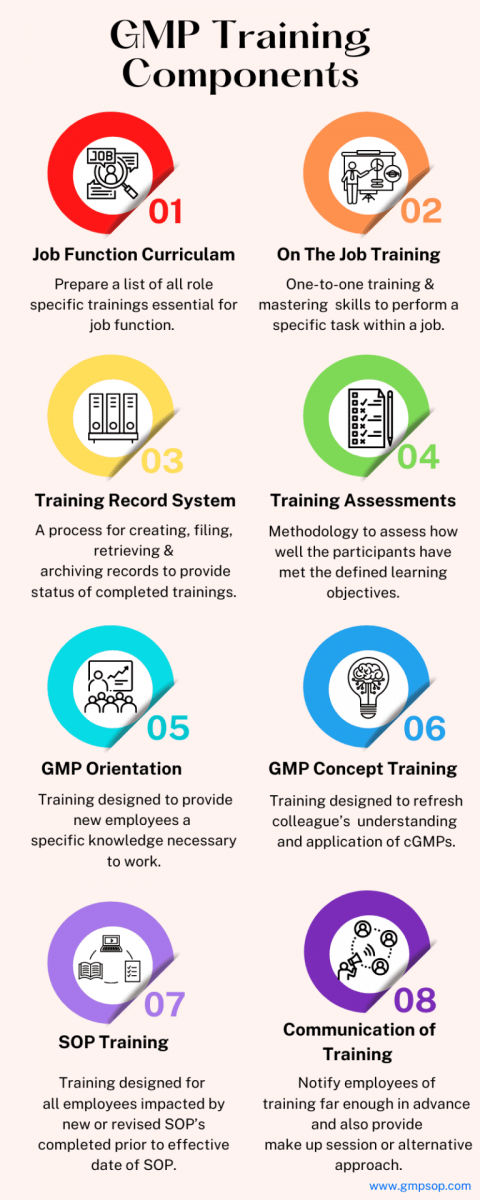
There are multiple ways training can be delivered. Some of those are listed in the beginning. However, types of training can vary business to business and their requirements respectively. Some of the common trainings are listed below:
Step 4: Training assessments
Instrument or methodology should be in place to gather feedback to improve the training process. For example, survey questionnaire should be requested the participants that measures training effectiveness relative to satisfaction.
Instrument or methodology should be introduced to measure learning assessment. The methodology may include:
Measurement to assess how well the participants have met the defined learning objectives and or links to business results.
– Measure trainee recall via written, verbal, computer based assessments, case studies, simulations or demonstrations
– Measure trainee performance via direct observation, feedback others, performance attributes or parameters.
– Measure impact of training on business performance by evaluating business results, metrics or data.
Step 5: Recording of training
A formal process for creating, filing, retrieving, and archiving training records should be developed that will track and report the status of completed and required trainings. Training records or reports must be readily available to substantiate that all employees performing GMP related functions have completed the training requirements.
Training officer and the department manager must store the training and assessment records to individual employee’s with the following details:
– Title of course and course code
– Date/s of course
– Trainer’s name
– Name of employees who attended
– Assessment results including the assessor’s signature
Step 6: Reporting of training
Typically, it is the role of training officer or administrator to training reports that include:
– Assess compliance of internal training programs to organizational requirements.
– Provide reports on current GMP compliance status for training.
– Maintain training records for QA.
– Compile performance KPIs (Key Performance Indicator) as required.
– Facilitate annual review of QA training requirements.
The training officer should also provide reports to management periodically with the following data:
– No. trainees trained on all procedures.
– No. trainees with training outstanding.
– A list of outstanding trainings for each trainee.
– A list of outstanding gowning qualifications.
– A list of outstanding Aseptic Practices Training.
Step 7: Employee awarding
As the trainings are completed, assessed and recorded, the responsible training office should update the skills training matrix. Employees are given cumulative scores on the matrix for a period typically one year based on their assessment performance.
It is important for the senior managers along with department managers to recognize the high scoring employees for their persistent hard work and reward appropriately for dedication and commitments.
Step 8: Archiving of training
All training records clearly showing employee name, role, course title, completion date, trainee signature, assessor name & signature, assessment score etc. should be archived after the departure of employee the workplace for a defined number years as required by the local regulatory authority.
Personnel data should be securely kept using physical means such as compactus or electronically by scanning the records and uploading into cloud based storage.

GMPc Vietnam is recognized throughout Vietnam as the leader in providing turnkey consulting solutions for GMP-certified facility projects, including Pharmaceuticals, Cosmetics, Health supplements and Veterinary pharmaceutical. Though 12 years of development 2011 to 2023, GMPc has implemented more than 230 GMP-certified facility projects, equaling to more than 80% of market share of the field in Vietnam. Not only do domestic customers, foreign investors also choose GMPc as their consultant when investing new factories in Vietnam, such as Kyoto Biken Vaccine Factory, Nippon Chemiphar Pharmaceutical Factory, Shimizu Contractor, Kajima Contractor, etc.
Consulting services for EU GMP
Consulting services by GMPc Vietnam
GMP project consulted by GMPc Vietnam


